The low down on SLS
There’s no shortage of ‘SLS-Free’ products on the market. With these free from claims, should we be avoiding SLS? What is it and what does science have to say? Let’s explore common claims made against SLS and what the science actually says, beginning with the basic question - what is SLS?
What is SLS?
Sodium lauryl sulfate (CH3)(CH2)11SO4Na), otherwise known as SLS (and also sodium dodecyl sulfate, or sodium coco-sulfate), is an anionic (negatively charged) cleansing surfactant made up of a 12-carbon tail and a polar sulfate ‘headgroup’ - this structure is what makes SLS an ‘amphiphilic’ (has fat and water-loving portions) surfactant/detergent (For a Surfactant Basics blog post, click here!). SLS is made by treating lauryl alcohol (derived from coconut or palm commonly but can also be petrol derived) with sulfur trioxide, followed by neutralization with sodium carbonate to produce the end surfactant. In your products, concentrations range from 0.01%-50%. The higher range is more commonly seen in household cleaning products, which don’t have the same irritancy concerns as a cosmetic product, plus they often need to be tougher cleansers. Note, just because this chemical is used for industrial cleaners does not mean that it’s going to be inherently bad for a cosmetic product - it’s the dose/concentration in the formula that matters… but more on that in a bit!
SLS is used in your products because it’s a dang good cleanser, it’s cheap, and it’s very easy to formulate with. For example, a common issue with SLS-free cleansers is that they are harder to thicken effectively. In contrast, add some betaine (usually in the form of Cocamidopropyl Betaine) to your SLS-containing formula with some salt and you can make that ‘perfect’ consistency in a pinch (literally).
It’s hard to pinpoint when all the concern for this ingredient started. For example, worries for carcinogenicity seemed to have arisen in the 90s, albeit largely from the media misinterpreting research. When SLS-free products came onto the market in the 2000s, originally with claims about stripping your hair, all of the sudden consumers became very aware of SLS in cosmetic products. In general, when people see a ‘free-from’ claim, they often make assumptions that the product is safer or better and that products with that ingredient will be bad… A big reason for the new regulatory guidance in the EU to make ‘Free-from’ claims (including SLS-Free) not okay - this change happened in July 2019.
So are there any good reasons to avoid SLS in your personal care routine? Is SLS safe? Below I’ve outlined the most common claims made against SLS and what the research actually says.
SLS is irritating.
Like most chemicals, yes, SLS is an irritant - SLS is one of the more irritating surfactants out there and can be seen used as a bench-mark irritant in studies… which you’ll see many agencies point to when this claim is made. But SLS isn’t used at 100% in personal care, it’s diluted into formulations. Also - Yes, studies have demonstrated that 24-hour exposure to 1-2% (w/w) solutions of SLS can cause more water loss through the skin and mild, but reversible, skin inflammation (note, irritation goes up with the SLS concentration and the length of exposure). Using all of this data to make claims about SLS in products though really just shows ignorance of how this ingredient is usually used - re, in wash-off products, usually in low percentages (below 10%), and in formulations that counteract irritancy. For example, combining SLS with co-surfactants (i.e. amphoteric (e.g. cocoamidopropyl betaine) and non-ionic (Coco or decyl glucoside) ones) and conditioning agents can produce extremely gentle products - flip your SLS containing shampoo bottle over and I can almost guarantee that you’ll see this in the ingredient list. In reality, SLS in a product does NOT mean that the product will necessarily be irritating compared to SLS-free products.
With all of this said, SLS is drying for some people due to its efficiency as a cleanser, so if you’re looking for a gentler cleanser, you can opt for things like sodium cocoyl isothienate or methyl cocoyl taurate, not quite as cleansing but still cleansing enough for products like shampoos, or in facial cleansers, surfactants such as glucosides or saponins (note, these alone will not be cleansing enough in a good shampoo product IMO). I see a lot of soap companies making fear-mongering claims about the irritancy of SLS in their marketing for why you should use their products…. just so you know, soap, due to its high pH (can’t be pH adjusted lower than about 9) and it’s tendency for soap scum, is often a lot more irritating than a well formulated product with SLS, which is pH adjustable.
Note, when I say soap, I mean the soap that’s made when you react sodium or potassium hydroxide with oils to produce solid or liquid soap (sometimes called castile soap if liquid). When correctly labelled, you’ll recognize these products with surfactants ‘sodium or potassium -oate’ on their ingredient lists (i.e. sodium olivate, sodium cocoate). There’s no shortage of mislabelled soaps though - sometimes you’ll also just see oils in the ingredient list or sometimes you’ll see oils + sodium or potassium hydroxide.
SLS can cause severe eye damage and blindness.
These claims often point to a study in the journal of Lens and Eye Toxicity Research by Green et al, which demonstrated that high SLS concentrations exposure to the eye can result in a slower healing process in response to damage (and in no way said that SLS causes blindness or severe damage in consumer products). In response to this media attention, Dr. Green’s (lead author of the study) legal counsel issued the following letter:
…your citation of his work was not simply a misinterpretation, it was plainly wrong. By citing his research in support of erroneous conclusions, you have libeled Dr. Green. In fact, [you have] even attributed quotations to Dr. Green which he has never written or spoken, and which he would not ever write or speak.
There’s not much more to say here other than this is a complete bunk claim. If you see someone saying this, this should be a big red flag.
SLS is toxic.
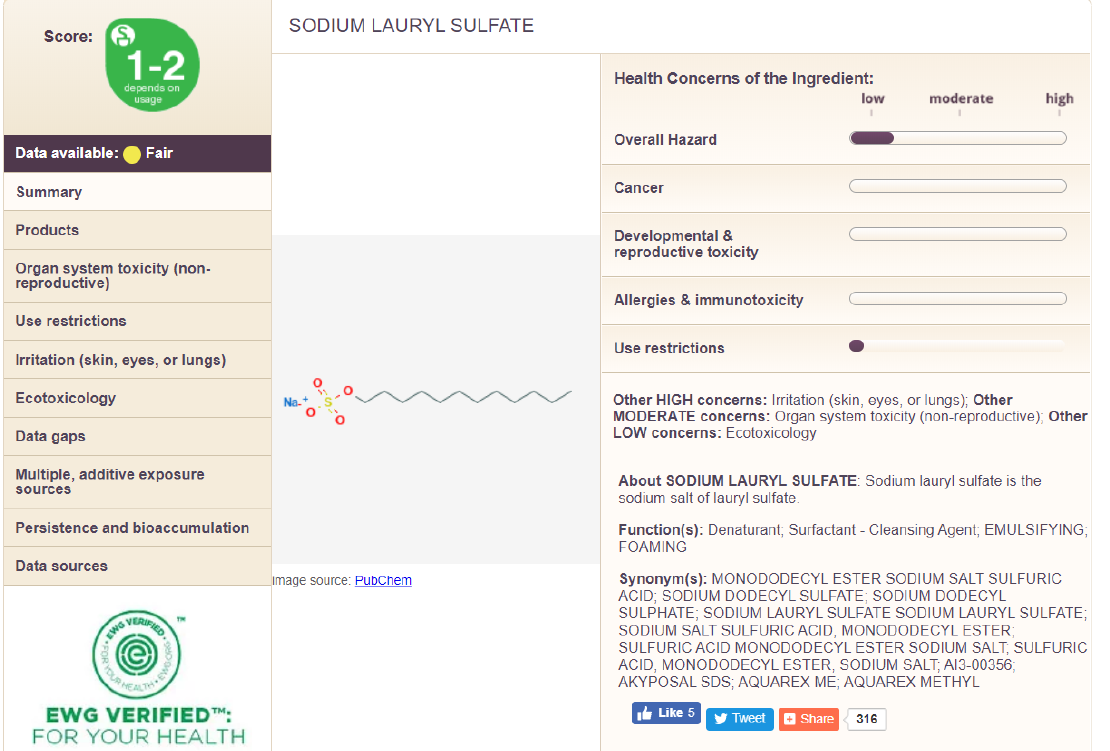
Something I hear a lot. For example, in the EWG, SLS is a ‘moderate concern’ linked to organ toxicity (for why the EWG is NOT a reliable information source, click here for my article ‘A Case Against the EWG’), so let’s dig into this a bit more - is SLS toxic? Acute oral toxicity refers to the immediate harmful impacts of ingesting something, measured in terms of median lethal dose (LD50), which indicates the quantity by weight needed to kill half of the laboratory animals getting that dose. Ingredients and formulations with an LD50 of ≥5,000 mg/kg are classified as non-toxic. The acute oral toxicity of SLS alone ranges from 600 to 1,288 mg/kg (in rats) - yes, SLS alone is toxic to rats. But here’s the thing, it’s the dose that makes the poison, and EVERY chemical has a toxic dose. For example, the LD50 of table salt (sodium chloride) is 3,000, making it moderately toxic by definition. In reality, when SLS is formulated into a product, such as in the case of your cosmetics, it can be considered non-toxic.
SLS is carcinogenic.
There is NO SCIENTIFIC EVIDENCE**** to support this. Moreover, SLS isn’t listed as a carcinogen in either the International Agency for Research on Cancer (IARC); U.S. National Toxicology Program; California Proposition 65 list of carcinogens; U.S. Environmental Protection Agency; and the European Union. I’ve seen 1,4-dioxane associated with SLS, which is categorized as possibly carcinogenic to humans by IARC… the potential for ethoxylated surfactants like SLES (sodium lauryl ether sulfate) to be contaminated from this process is well established… but non-ethoxylated surfactants, re- SLS, DO NOT share the same risk. Note, cross-contamination is still possible, but alas, 1,4-dioxane is also something tested and regulated in surfactants sold on the market today.
SLS absorbs into the blood and builds up in the heart, liver and brain.
Claims like this often will point to the Cosmetics Ingredient Review (CIR) Final Report on the safety of SLS. However, the CIR concluded that while SLS can be absorbed when applied directly, most of it remains on the skin surface. If it is absorbed, it’s quickly metabolized by the liver and rapidly excreted. There is NO evidence in this report, or in the scientific literature in general, to support the idea of organ accumulation and systemic toxicity. SLS DOES NOT bioaccumulate in humans.
SLS is an eco-toxin.
Aquatic toxicity refers to the short-term negative impacts to aquatic life from a chemical or finished product, also measured as median lethal concentration (LC50), with an LC50 of ≥100 mg/L classified as non-toxic to aquatic life. Alone, SLS has an LC50 between 1-13.9 ml/L after 96 hours, making it moderately toxic to aquatic life. Similar to oral toxicity, this doesn’t mean that formulated products will have this same risk, and in fact, SLS dilutions (re- products) are essentially non-toxic to aquatic life under most conditions. Moreover, by the time products reach natural waters, they’re largely degraded. Just to add, SLS is also readily biodegradable and does not persist in the environment.
And that’s a wrap! If you have a preference for SLS-free products - perhaps you prefer using cleansing with milder anionic cleansers, such as sodium cocoyl isethionate, or ‘naturally'-derived non-ionic ones, such as glucosides and saponins, then you do you. But if you like SLS containing products, don’t let SLS-free claims or fear-mongering on the ingredient spoil your opinion on this surfactant. Questions, queries, comments or concerns? Leave em’ below or on any of our social media feeds!
References
Bondi C et al. (2015) Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products.Environ Health Insights. 2015; 9: 27–32.
Cosmetic Ingredient Review (CIR). Final report on the safety assessment of sodium lauryl sulfate and ammonium lauryl sulfate. Int J Toxicol. 2005; 24(1): 1–102.
Green, K. Johnson, R.E. Chapman, J.M. Nelson, E. Cheeks, L. Preservative effects on the healing rate of rabbit corneal epithelium. Lens Eye Toxic Res. 1989; 6: 37–41.
Gloxhuber, C. Künstler, K. Anionic Surfactants: Biochemistry, Toxicology, Dermatology. 2nd ed., Vol. 43. New York: Marcel Dekker; 1992.
De Jongh, C.M. Verberk, M.M. Withagen, C.E. Jacobs, J.J. Rustemeyer, T. Kezic, S. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermatitis. 2006; 54(6): 325–33.
Horita K et al. (2017) Effects of different base agents on prediction of skin irritation by sodium lauryl sulfate using patch testing and repeated application test. Toxicology 382:10-15.
Lewis, M.A. Chronic and sublethal toxicities of surfactants to aquatic animals: a review and risk assessment. Water Res. 1991; 25(1): 101–13.
Karsa, D.R. Porter, M.R. , eds. Biodegradability of Surfactants. Glasgow: Blackie Academic & Professional; 1995.
Mizutani T et al. (2016) Sodium Lauryl Sulfate Stimulates the Generation of Reactive Oxygen Species through Interactions with Cell Membranes. J Oleo Sci. 65(12):993-1001.